TiCK1, ToCK2 goes the Circadian Clock
Subject areas: Molecular Biology
Vocabulary:
Circadian rhythms – any biological process that has a periodicity of about 24 hours, often coordinated with day-night cycles. These rhythms are endogenous, meaning they arise from the organism itself, not imposed upon them externally, but these biological clocks can be entrained, or “set” by outside influences. Circadian rhythms are observed in a wide range of organisms including bacteria, plants, and both invertebrate and vertebrate animals.
Phosphorylation – A key cellular process is phosphorylation, in which a phosphate group (a negatively charged group consisting of one oxygen linked to four phosphorous atoms) is added to or removed from an enzyme in order to activate or deactivate it. This is one of the most common and rapd ways to turn cellular mechanisms on and off, many times faster than producing more enzymes or destroying them. The enzyme that adds phosphate groups onto other molecules is called a kinase. The enzyme that removes phosphate groups is called a phosphatase.
The article:
Szabo, A. et al. (2013) The CK2 kinase stablizes CLOCK and represses its activity in the Drosophila circadian oscillator. PLoS Biol 11(8): e1001645. doi:10.1371/journal.pbio.1001645
Circadian clocks are fascinating. The idea that even inside very simple organisms there is an internally coded timer that reflects the periodicity of the earth’s rotation is pretty amazing. The oscillation of these internal biochemical reactions synchronizes myriad other activities in the organism, and disruption of circadian rhythms often has detrimental effects. Humans, for instance, can experience a common from of circadian disruption in jet lag, when the normal sleep cycle is disturbed by fast travel across multiple time zones.
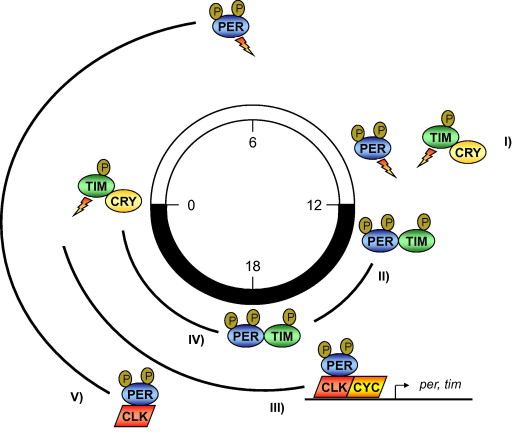
The circadian oscillations of the fruit fly, Drosophila, are known to include a molecular clock system that involves the products of the Period (PER), Timeless (TIM), Clock (CLK) and Cycle (CYC) proteins. The basic mechanism is that Clock and Cycle turn on the PER and TIM genes at the end of each day, which allows production and accumulation of Period and Timeless proteins. There is a negative feedback loop so that late in the night, the PER and TIM proteins have accumulated and are inhibiting CLK/CYC, thus turning off production of more PER and TIM. Through the day, PER and TIM are degraded, and by nightfall, the cycle can start all over again. Of course, this is only the basic mechanism – there are many other parts of the circadian clocks that can modulate the basic mechanism or be guided by it.
It has previously been shown that the kinase, CK2, can phosphorylate PER and TIM. This leads to their destabilization, and after a few other steps leading to degradation of the PER and TIM proteins, would eventually take away their inhibition of CLK/CYC. CK2 has also been shown to associate with in a complex with PER and TIM. Because of this and also because kinases that directly phosphorylate CLK have not been described yet, the authors of this paper investigated whether or not CK2 might be one.
Testing the hypothesis.
First, it is important to recognize that CK2 is actually a holoenzyme – composed of four subunits, two alphas and two betas. The CK2α sununits have the actual phosphorylating activity, while the CK2β units are regulators that usually increase phosphorylation by CK2. The first experiments this research group shows is that CK2α activity promotes phosphorylation of CLK, but it does not directly show that CK2 phosphorylates CLK. They also showed that CK2α activity was needed to stabilize CLK protein when PER and TIM were absent.
Both of these sets of experiments were carried out with flies that make a mutant dominant-negative form of CK2α. Dominant negatives are useful specific inhibitors of enzyme activity. Basically, they are almost like the normal protein, but without the enzyme activity. So, this mutant CK2α can bind onto a substrate, but not phosphorylate it. Essentially, it prevents the phosphorylation by hanging onto it instead of letting a normal enzyme get to it.
Unlike CK2α, it turns out that inhibiting CK2β does not have any effect on CLK stability. That isn’t very surprising since it does not have enzymatic activity of its own, but there was a possibility that it could modulate CK2α’s effect.
What do they need to show next? Proceeding methodically, they want to show that CK2α and CLK actually physically interact. We know one stabilizes the other, but it could be through intermediaries, so now we need to show they come in contact. They did this by co-immunoprecipitation. To put that long word in extremely simplistic terms, they ran a solution of fly head extracts (which is where the circadian clock proteins are in Drosophila) over a bed of tiny beads that have a tag that sticks to CK2α. All proteins run through the beads except for CK2α and whatever proteins are directly stuck to it. Then, they analyze whatever is stuck on the beads to see what they got (besides CK2α). As expected, they found CLK, but the neat thing is they found that it stuck to CK2α most strongly in fly extracts taken in the morning. So that’s cool – they think it’s part of a circadian clock, so it’s nice to see that there is a time-dependency to the interaction.
So what about the pure proteins in a test tube, taking away the many other proteins in the extract? It turns out that CK2, and CK2α in particular, can phosphorylate CLK in a pure in vitro setting. However, they also found that adding PER actually seems to enhance the phosphorylation significantly.
What they concluded.
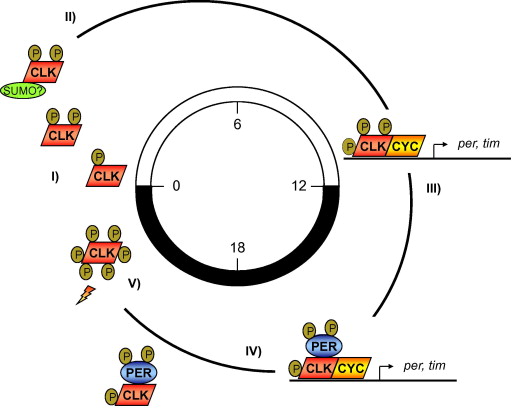
CK2 is definitely able to phosphorylate CLK. It does so better when PER is around, but CK2 activity also happens to destabilize PER. This may be a sort of control loop, automatically turning off CK2 from acting on CLK because it has no more PER. They also showed that the CK2beta subunits seem uninvolved in the regulation of CLK phosphorylation by CK2.
Oscillators like this usually are a constantly shifting balancing act between positive and negative actors. Previous work has shown that a related enzyme, CK1, destabilizes CLK. Thus, the cycle of CLK availability could be a balance of stabilization by CK2 and destabilization by CK1.
Why it’s interesting.
CK2 is a common protein across species, and circadian clocks are also a common phenomenom across species. Thus, insights into the regulation of the circadian clock in flies could have ramifications far afield. There could be ramifications on human health such as sleep and eating disorders. The ability to precisely alter the circadian clock may be crucial for acclimation in far future endeavors such as interplanetary travel and colonization.
The following diagrams are from Weber et al, FEBS Letters 585:1443 (2011). doi:10.1016/j.febslet.2011.04.008
The brown circled “P”s are phosphate groups. In the upper diagram, CK2 is likely the kinase that is phosphorylating the PER and TIM proteins. In the lower diagrams, we expect that the first three P’s on CLK are probably from CK2, because those are stabilizing/activating. On the other hand, we know that CK1 also acts on CLK and destabilizes, so that is probably responsible for the two further phosphorylations on CK2.

No comments
Be the first one to leave a comment.