Growth Factor Nasal Spray Repairs Mouse Brains
The paper: Scafidi, J. et al. (2014) Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature 506: 230-236. doi:10.1038/nature12880
Subject areas: neuroscience
Vocabulary:
oligodendrocyte – one of the non-neuronal cell types in the central nervous system (brain and spinal cord). Oligodendrocytes serve many different roles in supporting the function of neurons, including electrically insulating them with myelin.
—–
This article is a summary of a recent primary research paper intended for high school teachers to add to their general knowledge of current biology, or to supplement their lessons by showing students the kinds of projects that current biological research addresses.
—–
The brains of babies born very premature (under 8 months gestation) are highly susceptible to damage from hypoxia – lack of oxygen – because the lungs are not developed enough to draw in sufficient air or absorb enough oxygen into the body. This results in what is termed, diffuse white matter injury (DWMI) and is connected with a number of neurodevelopmental defects that can include behavioral and learning problems, vision and hearing impairment, and problems with motor control. Unfortunately, at this time there is little that can be done to treat the damage after it has happened.
What They Knew
Scafidi and colleagues have come up with a promising potential treatment for brain injury in babies that were born significantly premature. One of the interesting things about their approach, at least to the layman, is that they are concentrating not on the nerve cells (neurons) in the brain, but on support cells known as oligodendrocytes. Combining their previous work showing that epidermal growth factor receptors (EGFR) are important in oligodendrocyte development and work showing that disruption of oligodendrocyte maturation causes some aspects of DWMI, the researchers decided to test whether activating EGFR could help the cells recover from hypoxic injury.
One of their observations was that there was an increase in epidermal growth factor (EGF) levels in white matter after chronic hypoxia. Is the increase in EGF mean that the injured tissue is producing more in order to activate the EGF receptors?
What They Did
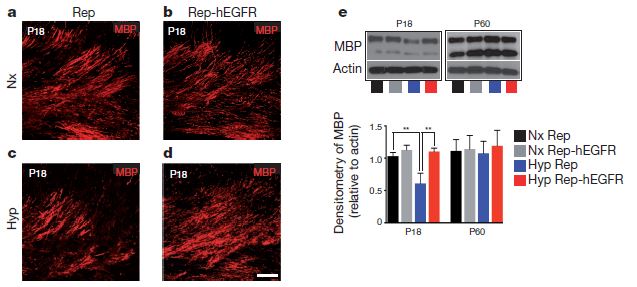
One of the first experiments was to observe changes in oligodendrocyte maturation in either normal mice or EGFR-overexpressing mice in hypoxic conditions. It turns out that having more EGF receptor being expressed in oligodendrocyte precursors actually helped to prevent some of the markers of DWMI. The figure below shows the difference in myelin basic protein expression in normal (a and b) and hypoxic (c and d) conditions in normal (a and c) and EGFR-overexpressing (b and d) mice.
Hypoxia clearly decreases MBP expression in normal mice (c) but has less if any detectable effect on the micr expressing extra EGF receptor. Part (e) of the figure shows the amount of MBP that could be purified from preparations of white matter under the various conditions. In the young (18-day-old, P18) mouse, there is a clear and significant drop in MBP under chronic hypoxia (blue), but the same lack of oxygen does not trigger MBP loss in animals expressing high levels of EGFR. The P60 side just shows that there is eventually recovery of oligodendrocytes by 60 days postnatal.
Similar effects were seen with apoptosis. Normal mice that were placed in a chronic hypoxic state had an increase in apoptosis (cell death) in oligodendrocyte precursor cells at P11 and P18. However, the increased apoptosis was not observed in the EGFR-overexpressing mice. Furthermore, EGFR-overexpressing mice increase the proliferation of oligodendrocyte precursors in both normoxic (normal oxygen levels) and hypoxic conditions.
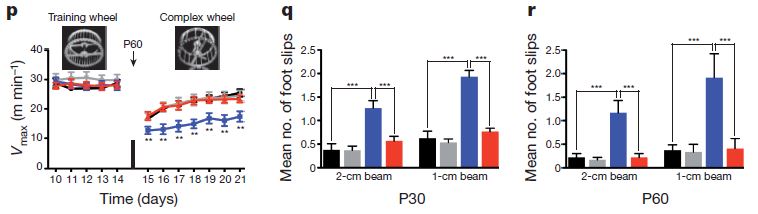
So there is improvement at the cellular level – but that is medically useless if it does not translate into improved function. The panel below shows the results from two kinds of functional experiments.
In (p) the mice run on exercise wheels. They were first trained to run on normal wheels with 38 rungs of the circular “ladder” and then confronted with the task of running on a wheel that had 22 of the rungs removed in an alternating pattern. Not surprisingly, the mice were all slower on the complex wheel, but the hypoxic mice without EGFR-overexpression were significantly slower at the beginning of the challenge and continued to lag behind through the observation period. Similarly, in (q) and (r), the mice were challenged to walk on 1-cm and 2-cm wide beams at a 30-degree incline. The graph shows the number of times the mice’s feet slipped during the task. Again, EGFR-overexpressing mice did just as well in hypoxic conditions as in normal-oxygen, but control mice were significantly less sure of foot when chronically hypoxic.
Together, these data mean that when there are more active EGF receptors, the damage from chronic hypoxia can be lowered. Unfortunately, getting cells to make more EGF receptors is a rather difficult task and at present is not clinically practical. It essentially requires gene therapy techniques that are still in development and very limited. But, what if instead of trying to get the cells to make more EGF receptors, they just over-activated or constantly activated the receptors they already have?
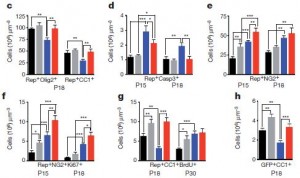 Because previous studies have shown that intranasal delivery of drugs to the brain was possible, it seemed that a very simple and clinically possible method of delivering extra EGF to the brain was possible. In fact, as these graphs show, treatment with EGF in hypoxic conditions (red bars) clearly increases the number of oligodendrocyte precursors (c), decreases apoptosis (d), increased oligodentrocyte precursor proliferation and differentiation into ogodendrocytes (e-h) compared to control untreated mice in hypoxic conditions. In normal oxygen conditions EGF-treated (grey) also has an enhanced proliferation effect on non-treated (black), but does not affect apoptosis.
Because previous studies have shown that intranasal delivery of drugs to the brain was possible, it seemed that a very simple and clinically possible method of delivering extra EGF to the brain was possible. In fact, as these graphs show, treatment with EGF in hypoxic conditions (red bars) clearly increases the number of oligodendrocyte precursors (c), decreases apoptosis (d), increased oligodentrocyte precursor proliferation and differentiation into ogodendrocytes (e-h) compared to control untreated mice in hypoxic conditions. In normal oxygen conditions EGF-treated (grey) also has an enhanced proliferation effect on non-treated (black), but does not affect apoptosis.
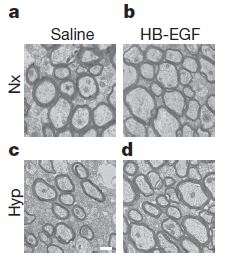 This is mirrored in electron micrographs (at left) depicting cross-sections of axons in the brain. EGF treatment increases axon size and number slightly in normal oxygen (Nx), but in chronic hypoxia, EGF significantly increases the size and number of neurons over the untreated saline control (c), which shows a large decrease in botht he number and size of the axons.
This is mirrored in electron micrographs (at left) depicting cross-sections of axons in the brain. EGF treatment increases axon size and number slightly in normal oxygen (Nx), but in chronic hypoxia, EGF significantly increases the size and number of neurons over the untreated saline control (c), which shows a large decrease in botht he number and size of the axons.
Finally, there is also functional improvement. Here, the red line shows that the ability of hypoxic animals to use the complex exercise wheel is significantly better with EGF treatment than without EGF treatment (blue line). The black line shows performance in normal oxygen conditions without EGF treatment.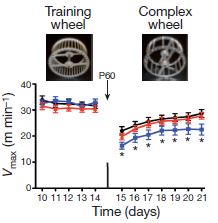
Of course, this is only being done in mice, which have remarkably resilient nervous systems compared to humans, and not in prematurely born mice, so while promising, it is only an early exploratory step in trying to find an effective treatment for diffuse white matter injury in very premature babies.

No comments
Be the first one to leave a comment.