Hibernation and (lack of) Muscle Wasting
The paper: Xu, R. et al. (2013) Hibernating squirrel muscle activates the endurance exercise pathway despite prolonged immobilization. Experimental Neurology 247: 392-401. doi:10.1016/j.expneurol.2013.01.005
Subject areas: Physiology
Vocabulary:
Skeletal muscle – a striated muscle usually composed of a mix of fiber types, including Type I (slow-twitch), Type IIa (moderately fast), and Type IIb (very fast). There are a few exceptions: the muscles that move the eyeball are completely fast-twitch fibers, while some postural muscles of the back are solely made of slow-twitch fibers. The ratio of fiber types can change depending on the kind of exercise regularly demanded of the muscle. Muscles are tissues composed of muscle fibers, which are each individual elongated cells. Unlike most cells, they have more than one nucleus, because they actually developed from a fusion of multiple precursor cells.
—–
This article is a summary of a recent primary research paper intended for high school teachers to add to their general knowledge of current biology, or to supplement their lessons by showing students the kinds of projects that current biological research addresses.
—–
We revisit hibernation today, but instead of the brain, the focus is on the muscles. We have also moved from the freshwater turtle to a small mammal, the 13-lined ground squirrel, Ictidomys tridecemlineatus. You are probably aware, either from personal experience or through someone you know, that if you stop or severely limit movement of a muscle (e.g. a broken arm in a cast), the muscle very quickly begins to atrophy, losing both mass and strength. This is true, not just for us humans, but for other animals as well. Given that hibernation is a prolonged state of little or no motion, not to mention low metabolism and low oxygen, the fact that hibernating animals are able to keep a large proportion of their muscle mass is an interesting mystery. The authors of this paper attempt to answer the question of how hibernating ground squirrels keep their muscle mass through the winter.
The 13-lined ground squirrel is found in the Great Plains of North America. On average, the ground squirrels enter hibernation in November/December, and remain that way until April/May. The animals experience an approximately 97% drop in heart rate during hibernation (from 300 beats per minute to just 5-10bpm), and lower their body temperatures to ambient temperatures, even near freezing. Every 3 weeks during hibernation, the animals rouse very slightly, at which point shivering helps to generate body heat back up to normal, then they return to full hibernating torpor after a few hours. They do not eat or drink during these semi-awake periods. For the experiments, once the squirrels showed signs of preparing to enter hibernation, they were moved to housing in a continuously dark environment maintained at 4 ºC.
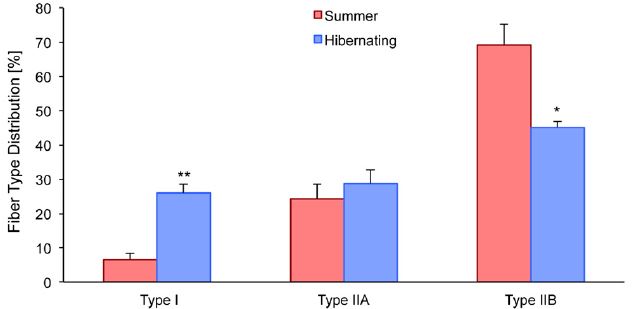
Muscle tissue samples were obtained from both hibernating and non-hibernating squirrels. The first analysis was to determine if there was any difference in the makeup of the muscle. Indeed, there was a significant change in the fiber type distribution in the quadriceps (upper leg muscle), with a sharp increase in Type I slow-twitch fibers, a drop in Type IIb fast-twitch fibers, and no change in Type IIa. So, there is an overall shift toward more slow-twitch fibers, which means, somewhat unexpectedly, that the muscle becomes more oxidative.
As a reminder, fast-twitch muscles produce the cellular energy carrier, ATP, quickly using glycolysis without oxygen to allow for quick movements, but fatigue quickly. Endurance activities rely on slow-twitch muscles, which take longer to produce ATP (using oxidative phosphorylation and requiring oxygen). Although one might argue that increasing slow-twitch muscles makes functional sense than keeping fast-twitch fibers that aren’t really needed in hibernation, it is still a little unexpected because there is very little oxygen available in hibernation with the heart rate so slow, and a switch to fiber types that do not require oxygen could make sense in that way.
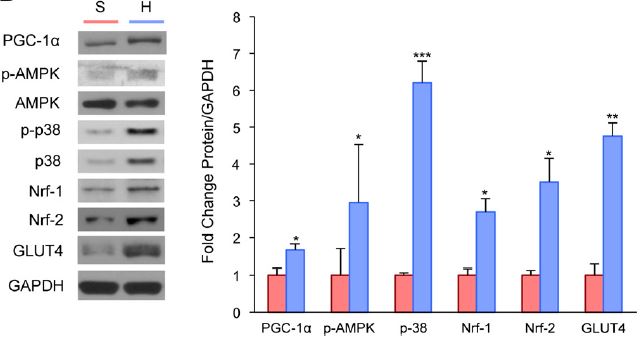
Xu et al then delved into the molecular mechanisms behind the switch. Other work on muscle remodeling, particularly in response to exercise, showed that adaptations to endurance exercise are mediated by PGC-1α. This molecule, more completely named peroxisome proliferator-activated receptor gamma coactivator 1-α, regulates mitochondria formation in skeletal muscle and formation of Type I muscle fibers. The known PGC-1α signal cascade also includes PGC-1α activators AMP-activated protein kinase (AMPK) and p38 MAP kinase, as well as Nrf-1 and Nrf-2 which are downstream targets of PGC-1α. Nrf-2 helps to activate upregulation of GLUT4, which is important in getting glucose into the cells. The investigators took the muscle samples, and this time looked for changes in the amounts of these different proteins in hibernating muscle. The figure below clearly shows a significant increase in each of these proteins. The GAPDH shown at the bottom of the protein blots part of the figure is just a control to show that the same overall amount of protein is loaded in each experiment.
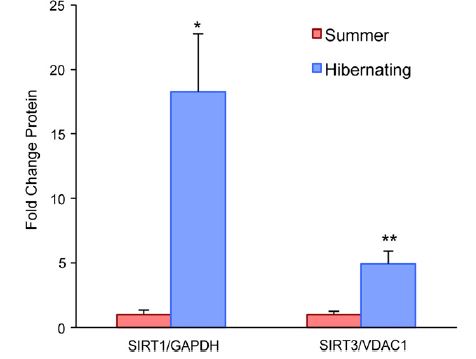
Interestingly, even though there is a switch to a more oxidative fiber type, there is no sign of oxidative stress in hibernating muscle. This can be explained by the upregulation of antioxidant stress response and anti-apoptotic (cell death) signaling during hibernation. The proteins shown here are all part of the mitochondrial antioxidation response. 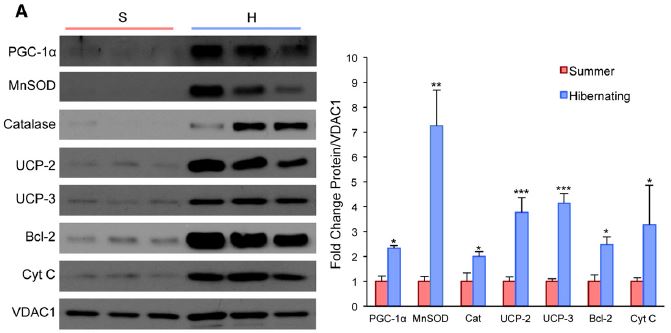 There are also more mitochondria in general in muscle from hibernating animals. Finally, the researchers also show that there is an increase in sirtuin levels. Sirtuins are proteins that have been implicated in activating PGC-1a after endurance exercise, as well as increasing mitochondria biogenesis.
There are also more mitochondria in general in muscle from hibernating animals. Finally, the researchers also show that there is an increase in sirtuin levels. Sirtuins are proteins that have been implicated in activating PGC-1a after endurance exercise, as well as increasing mitochondria biogenesis.
Together, these findings suggest that hibernation somehow activates an endurance-exercise muscle maintenance pathway without any actual exercise. This entails a relative increase in slow-twitch fibers and a decrease in fast-twitch fibers, concurrent with an increase in the number of mitochondria within the muscle fibers. Most of the molecular markers of the endurance-exercise pathway are activated, indicating this is likely the same pathway activated by something other than exercise, as opposed to coincidental use of a few components of the pathway.
Given the many different conditions that can lead to muscle atrophy from disuse in humans, gaining insight to alternate pathways to muscle maintenance could have significant clinical value.


No comments
Be the first one to leave a comment.