Pain and Pain Suppression Go Hand-in-Hand
The paper: Corder, G. et al. (2013) Constitutive µ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341: 1394-1399. doi:10.1126/science.1239403
Subject areas: Neuroscience
Vocabulary:
Hyperalgesia – abnormal sensation of pain from stimuli that should not cause pain, or excessive pain from stimuli that usually cause milder levels of pain.
Nociception – the nervous system mechanisms and connections involved in perception of pain. Nociceptive neurons are the nerve cells that detect noxious stimuli and send a signal of pain to the brain.
—–
This article is a summary of a recent primary research paper intended for high school teachers to add to their general knowledge of current biology, or to supplement their lessons by showing students the kinds of projects that current biological research addresses.
—–
Let’s try something a little different this time. We’ll start by breaking down the title. “Constitutive μ-opioid receptor activity” needs to start with the idea of a receptor. As the name implies, a receptor is a molecule that receives a signal from another cell (or sometimes itself). When a receptor, often on the surface of the cell, receives the correct molecular signal, it becomes activated. That generally means it changes its shape, and in doing so, it turns on some kind of enzyme activity. This enzyme may be a part of the receptor itself or one that is hooked up to it, in either case, it is only turned on when the receptor has been activated by binding the specific signal, or ligand. Except… the adjective, “constitutive,” here means that the receptor is constantly turned on, independent of whether ligand is available.
OK, so we have a receptor that is constantly turned on. It happens to be a μ-opioid receptor, so its normal ligand is a molecule in the opioid family – familiar ones are morphine, codeine, oxycodone, and hydrocodone – all of which have pain-relieving qualities. Continuing on, “endogenous analgesia” means that the body is itself providing analgesia, or pain relief. That sounds good, unfortunately, the last part of the title is “and dependence.”
The researchers were testing the hypothesis that injury can increase constitutive μ-opioid receptor activity, which could then lead to dependence at a cellular level.
The first set of experiments involved the induction of pain in mice by injecting an irritant into the footpad of the left back paw. Usually, this causes the paw to be hyperalgesic (extra sensitive to pain) for about ten days. However, if the mouse is given naltrexone hydrochloride(NTX) the hyperalgesia continued as long as the NTX was given, up to 14 days. NTX has no effect on control mice (who just had a saline injection).
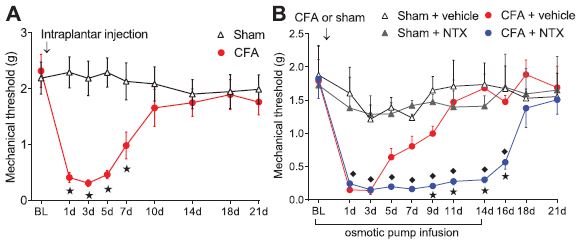
Mechanical threshold means the amount of force needed to cause the mouse to pull its paw away from the filament poking it. “Sham” refers to an injection of saline, compared to “CFA” which is an injection of the irritating substance, Complete Freund’s Adjuvant.
The closely related drug, NMB (naltrexone methobromide) did not prolong the pain. What does this mean? NTX and NMB are opioid receptor antagonists, meaning that they bind onto the opioid receptor, but instead of activating it, they prevent it from being activated. The difference between NTX and NMB is that NTX can pass the blood-brain barrier into the brain and spinal cord. Together, the experiments suggest that the diminishing of pain over time is at least partly due to activation of opioid receptors in the brain or spinal cord.
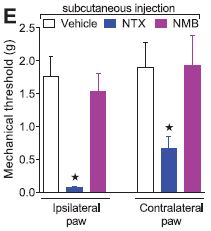 This is also supported by the finding that not only is the injected paw hypersensitive, but the contralateral paw is also, though to a lesser extent. This likely means that the sensitization process is not just happening in the nerves leading from the paw to the spinal cord. In fact, the photomicrograph below shows an image of pERK staining in the ipsilateral (injected) and contralateral (uninjected) sides of the dorsal horn of the spinal cord. ERK is an enzyme activated in the opioid receptor pathway, and pERK is the activated form. At 21 days post-injection, ERK is not activated by light touching of the injured paw. But, as the image shows, when NTX is introduces, light touching increased pERK staining in the spinal cord areas corresponding to both rear paws.
This is also supported by the finding that not only is the injected paw hypersensitive, but the contralateral paw is also, though to a lesser extent. This likely means that the sensitization process is not just happening in the nerves leading from the paw to the spinal cord. In fact, the photomicrograph below shows an image of pERK staining in the ipsilateral (injected) and contralateral (uninjected) sides of the dorsal horn of the spinal cord. ERK is an enzyme activated in the opioid receptor pathway, and pERK is the activated form. At 21 days post-injection, ERK is not activated by light touching of the injured paw. But, as the image shows, when NTX is introduces, light touching increased pERK staining in the spinal cord areas corresponding to both rear paws.
Interestingly, in an extension of that experiment, the authors discovered that if they introduced NTX after 21 days (over ten days after pain has disappeared), it would induce significant pain hypersensitivity, not just to touch, but also to heat. In fact, NTX also appeared to induce spontaneous pain even without a touch on the paw. This seems to show that even after pain is apparently gone, the reason could be that it is only being blocked by the body activating its own opioid receptors. This was consistent across a number of different tests, from a simple touch test, poking the irritated paw with varying degrees of pressure, to behavioral tests.
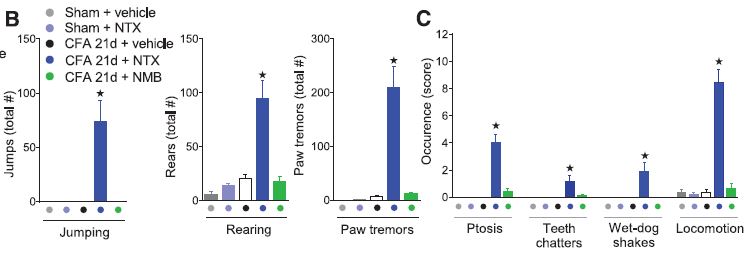
Two observations in particular stand out. The re-activation of hyperalgesia by NTX could be done even three months after the injection, suggesting a very long-term engagement of constitutive opioid receptor activity. 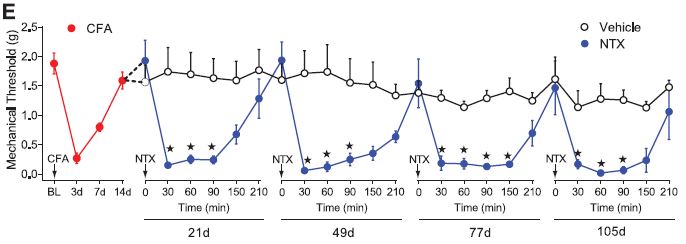 Also, in the behavioral tests, NTX elicited symptoms that mirrored those of classic morphine-withdrawal symptoms. Further study using CTOP and β-funaltrexamine, which are both like NTX but only act on a specific type of opioid receptor, showed that μ-type opioid receptors are the mediators of this phenomenon.
Also, in the behavioral tests, NTX elicited symptoms that mirrored those of classic morphine-withdrawal symptoms. Further study using CTOP and β-funaltrexamine, which are both like NTX but only act on a specific type of opioid receptor, showed that μ-type opioid receptors are the mediators of this phenomenon.
It also appears that another receptor signaling pathway is involved. The NMDA (N-methyl-D-aspartate) receptor-calcium-dependent pathway was tested with similar pain-inducing procedures, and Ca++ concentration and the enzyme adenylyl cyclase 1 (AC1) was activated. This pathway and adenylyl cyclase 1 in particular are known to be involved in morphine dependence and withdrawal. AC1 in the brain has also been implicated in chronic pain.
Beyond its title, this paper importantly shows that tissue injury and pain can initiate a long-term blockade of some pain signals, but concurrently and paradoxically activates a sensitization pathway that primes the nervous system for hyperalgesia, not just in the area of an initial injury, but possibly other parts of the body as well. If this is the case, one implication is that a loss of constitutive μ-opioid receptor activity could lead to chronic pain. Considering chronic pain is afflicts 100 million Americans (according to a 2011 Institute of Medicine report), and as many as 1.5 billion people worldwide, understanding the mechanisms behind this often frustratingly difficult illness could be invaluable.


No comments
Be the first one to leave a comment.