DNA should not be in the cytoplasm.
Subject areas: Immunology, Cell Biology
Vocabulary:
transfection – introducing DNA into a cell by physical means. Two common methods are lipofection, in which the DNA is packaged into tiny lipid vesicles that fuse with the cell membrane to release the DNA inside, and electroporation, in which a high voltage is passed through the cells, causing their membranes to open many micro-tears for a very short amount of time, which allow DNA that was in the growth medium to come inside.
cytokines – molecular messengers, usually small peptides, that have an effect on the immune system. There are many different cytokines, some of which may mediate an inflammatory response, while others may oppose it. Because some cytokines have effects throughout the body, there is some overlap in definitions between hormones and cytokines.
The article:
Li, X-D. et al. (2013) Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341(6152):1390-94. doi:10.1126/science.1244040
Eukaryotic cells like our own are characterized by compartmentalization into organelles. The genetic material is mostly stored in the nucleus, where it can be copied but otherwise kept away from all the other activity inside the cell. Presumably, this helps to protect it; it also keeps the very large charged macromolecule out of the way of everything else. There is also a little bit of DNA inside the mitochondria, mostly because the mitochondria evolved from a separate organism eons ago, and some of its genetic material continues to be made and maintained internally. We do not find DNA in the rest of the cell. In fact, the presence of DNA in the cytoplasm would mean one of two things: either the cell was damaged or sick to the point the DNA was leaking out of the nucleus or mitochondria, or the cell had been attacked by a virus carrying DNA.
Since DNA in the cytoplasm of a cell is a strong indicator of something wrong, it stands to reason that organisms may have evolved a mechanism for sensing this, and that it may then trigger some kind of response to limit the damage to either the cell itself or to those nearby. In fact, in mammalian cells, DNA inside the cytoplasm will cause the secretion of interferons and other cytokines that regulate immune responses.
What they knew.
From previous work, this group determined that cGAS is a cytoplasmic protein that binds to DNA molecules in vitro. cGAS is an enzyme that produces 2’3’cGAMP from a fusion of the nucleotides cGMP and cAMP connected via each others’ phosphate groups and sugars (see figure below). They also showed that the cGAMP that was produced could then activate a a series of proteins that could lead to cytokine production.
In this paper, they wanted to see if the conclusions from their in vitro experiments could be demonstrated and extended in vivo.
What they did.
First, they generated cGAS knockout mice. These are mice in which the gene that produces cGAS is altered so that the protein is truncated and nonfunctional. Interestingly, the knockout mice appear to develop normally despite missing the gene. Now that they have a source for cGAS-less cells, it was time to test them.
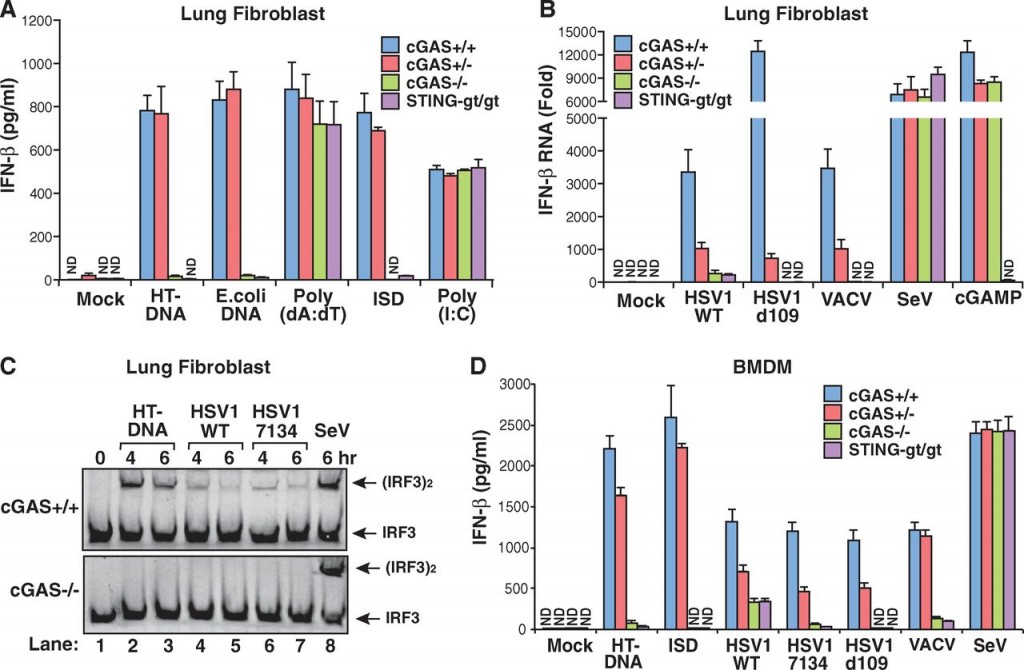
Lung cells from normal wild-type mice, cGAS knockouts, cGAS +/- heterozygotes and goldenticket mice were cultured and then tested with several different types of DNA (figure 1A, below). The DNA was mixed with a solution that encapsulated it into tiny lipid spheres that can fuse with the cell membrane and release their contents into the cytoplasm. As expected, two types of natural DNA (from herring testis and E. coli) caused wild-type mice to initiate a reaction that led to secretion of the cytokine, interferon-beta (IFN-β). The cGAS knockouts did not secrete any IFN-β, nor did the goldenticket mice, which are mutants that have the gene for STING knocked out. STING would normally bind to and be activated by cGAMP produced by cGAS, and then go on to activate enzymes that help start IFN-β production. The heterozygote, which has one good copy of the cGAS gene and one knockout copy, was still able to secrete IFN-β.
The only DNA-induced IFN-β that was not decreased by cGAS knockout was an artificial DNA construct of repeated adenines and thymines (Poly A:T).
As a control, they treated the cells with just the transfecting lipid without DNA (Mock), a double-stranded RNA molecule (Poly I:C) that induces IFN-β production by a different pathway. Knocking out cGAS did not affect IFN-β production in either of these cases.
Most of this is what they expected, and supported the idea that cGAS is needed for cells to respond to foreign DNA in the cytoplasm by activating cytokine production to call in the immune system.
They next checked (Fig. 1B and 1D above) lung cells and bone marrow-derived macrophages from the various mice for their ability to engage IFN-β production in response to exposure to a number of DNA viruses including herpes simplex I (HSV1), a mutant HSV1 (d109) that is missing proteins that antagonize immune response, and vaccinia (VACV), a virus related to cowpox and smallpox viruses. They also tested Sendai virus (SeV) which is an RNA virus and does not inject DNA into the cytoplasm. Again, knocking out cGAS blocked the IFN-β production in all the DNA viruses, but did not affect IFN-β production initiated by RNA virus infection.
The authors also tested other cell types, as well as the ability to initiate interferon-alpha (IFN-α) production in those cell types that normally do so. Again, knocking out cGAS seems to abolish the cytokine production response.
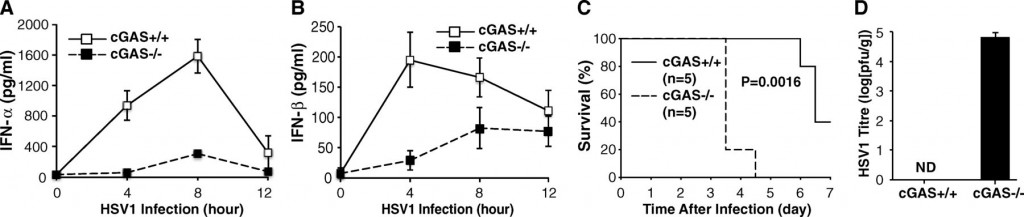
Finally, they took the experiment from cell cultures to the live animal, and infected wild-type and cGAS-knockout mice with the herpesvirus. The figure below shows that not only are interferon-α and -β levels significantly lower in cGAS knockouts (A and B), survival of the knockout mice is drastically cut (C).
What they learned.
Li et al confirmed that the observations they made with protein preparations were in fact true in vivo, both in cultures of specific cell types and living animals. There appears to be a clear pathway in which DNA in the cytoplasm is bound by cGAS. This activates the cGAS enzyme, which then makes a bunch of cGAMP, each of which binds to STING. STING, then activates a pair of kinases that then activate transcription factors that can turn on production of cytokines such as interferon-alpha and interferon-beta.

No comments
Be the first one to leave a comment.