Making Bacterial Assassins
Subject areas: Microbiology, Synthetic biology
Vocabulary:
Chemotaxis – the movement of cells toward or away from particular chemicals, including nutrient sources or noxious substances.
Biofilm – biofilms are an often-sticky dense matrix of secreted proteins, nucleic acids, and carbohydrates produced by bacterial colonies as a protective measure. Antibiotics have difficulty penetrating the biofilm to reach the bacteria themselves, and because the biofilm is often gelatinous and resilient, it also protects the bacteria from physical disruption as well. Perhaps the most well-known biofilm is the plaque that forms on our teeth.
The article: Hwang, I.Y. et al. Reprogramming microbes to be pathogen-seeking killers. ACS Synthetic Biology (2013). doi:10.1021/sb400077
The Goal
Hwang et al have undertaken a synthetic biology project to transform ordinary gut bacteria, Escherichi coli, into an assassin, seeking out and killing the pathogen, Pseudomonas aeruginosa. A very common bacteria, P. aeruginosa can thrive not only in soil and water, but on animals and plants, and even artificial environments, like plastics. It can also form a biofilm, which makes eradication more difficult by shielding the organisms from some antibiotics and even from attack by immune system cells. It is not usually a cause for concern in healthy individuals, but it can be a serious opportunistic infection in people with weakened immune systems (often from another disease).
E. coli is not a natural competitor or killer of P. aeruginosa. However, it is a very well-characterized bacteria that molecular biologists have worked with for a long time. So, the researchers tried to give it the characteristics needed to find the Pseudomonas and secrete something to kill them.
Technical Challenges
Getting cells to make and secrete stuff is nothing new. However, designing a mechanism to secrete only when in the vicinity of the target would be a novel challenge. The E. coli would need a way to find or sense Pseudomonas, and a method to move to them.
Another challenge is to be able to attack the target cells both in their single-cell forms and also when they are part of a biofilm. Whatever the killer cell secretes needs to be able to penetrate or destroy biofilms as well as kill the target cells.
Genetic Circuit
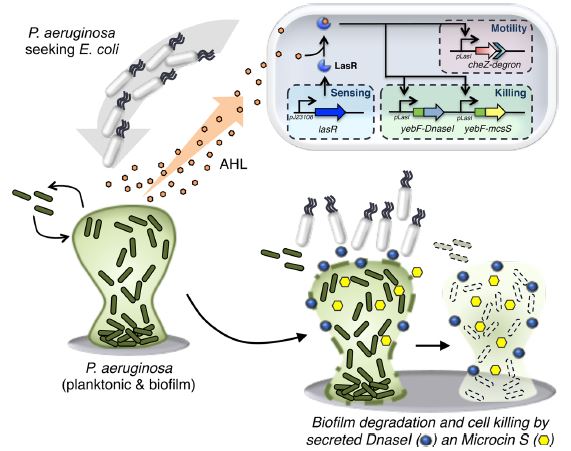
The researchers inserted a genetic circuit into the E. coli genome that works as shown in the figure below. AHL is N-Acyl homoserine lactone, which is a compound secreted by P. aeruginosa, but not E. coli. This is picked up by LasR proteins in the E. coli which then activate LasR-AHL-sensitive promoters to turn on three separate pathways. One is for moving towards the source of AHL. Another is for production of DnaseI, which helps break down biofilms. And the third is for production of Microcin S, which kills the P. aeruginosa.
This uses the molecular biology concepts of promoters and activators. You have some idea of what a gene is: it’s just a stretch of DNA that encodes something the cell uses, like a protein. You probably also intuitively realize that your cells are not constantly using all of your genes all the time to make everything. There is a sequence of the DNA just before you get to the part that actually codes for something called the promoter. It is the launchpad for using the DNA to make something, and all the enzymes and associated proteins have to come together around the promoter before they can be turned on to make the gene product. An activator is one of those associated proteins that lands on the DNA around the promoter sequence, and helps direct the important enzymes into the right place.
In the work here, the scientists have inserted genes for the Dnase I, Microcin S, and cell movement (sort of, more on that later) into the killer E. coli, all using the same promoter, and therefore all able to use the same activator. The activator is a protein, LasR, that does not actually function as an activator until it binds onto AHL. Then it changes shape, becomes and functional activator, and jumps onto the promoters to make stuff.
Where are we going?
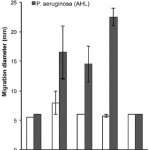 E. coli normally display chemotaxis toward food sources. However, the E. coli used here are deltacheZ mutants that have a deletion in the cheZ gene, which disrupts chemotaxis, causing the bacteria to tumble about in place without going anywhere. By putting in a working cheZ gene under the control of the pLasI promoter, the availability of AHL (secreted by the target P. aeruginosa) turns chemotaxis back on, leading the E. coli towards the targets. They had to fiddle with the gene a little because too much CheZ can actually turn chemotaxis off again. Still, the graph here shows that none of the strains showed much movement towards an E. coli target (white bars) but the ones with the inserted CheZ genes showed significant movement towards P. aeruginosa targets (grey bars). The far right represents wild-type E. coli that are not deltacheZ mutants but also do not have CheZ inserts.
E. coli normally display chemotaxis toward food sources. However, the E. coli used here are deltacheZ mutants that have a deletion in the cheZ gene, which disrupts chemotaxis, causing the bacteria to tumble about in place without going anywhere. By putting in a working cheZ gene under the control of the pLasI promoter, the availability of AHL (secreted by the target P. aeruginosa) turns chemotaxis back on, leading the E. coli towards the targets. They had to fiddle with the gene a little because too much CheZ can actually turn chemotaxis off again. Still, the graph here shows that none of the strains showed much movement towards an E. coli target (white bars) but the ones with the inserted CheZ genes showed significant movement towards P. aeruginosa targets (grey bars). The far right represents wild-type E. coli that are not deltacheZ mutants but also do not have CheZ inserts.
Does the killer cocktail work?
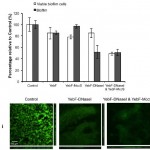 Although it is not completely effective, the secreted DnaseI/Microcin S clearly destroys the biofilm barrier and kills the Pseudomonas cells embedded within. YebF is a small secreted protein normally made by the E. coli, but in this case is fused to the genes for DnaseI and Microcin S, to help transport them out of the cell. From left to right, the bar graph shows the control of P. aeroginosa biofilms incubated with non-engineered E. coli. The next set is mixed with E. coli secreting extra YebF, which is also a sort of control since the other engineered E. coli will be secreting some along with the other inserted genes. There is a slight decrease in live cells and biofilm material, but not statistically significant (note error bars). Adding Microcin S – secreting cells kills some without affecting the amount of biofilm, while DnaseI by itself chops down a lot of biofilm but does not significantly kill cells. Finally though, the combination cocktail kills about half the cells and knocks down the amount of biofilm as well.
Although it is not completely effective, the secreted DnaseI/Microcin S clearly destroys the biofilm barrier and kills the Pseudomonas cells embedded within. YebF is a small secreted protein normally made by the E. coli, but in this case is fused to the genes for DnaseI and Microcin S, to help transport them out of the cell. From left to right, the bar graph shows the control of P. aeroginosa biofilms incubated with non-engineered E. coli. The next set is mixed with E. coli secreting extra YebF, which is also a sort of control since the other engineered E. coli will be secreting some along with the other inserted genes. There is a slight decrease in live cells and biofilm material, but not statistically significant (note error bars). Adding Microcin S – secreting cells kills some without affecting the amount of biofilm, while DnaseI by itself chops down a lot of biofilm but does not significantly kill cells. Finally though, the combination cocktail kills about half the cells and knocks down the amount of biofilm as well.
Can they hunt?
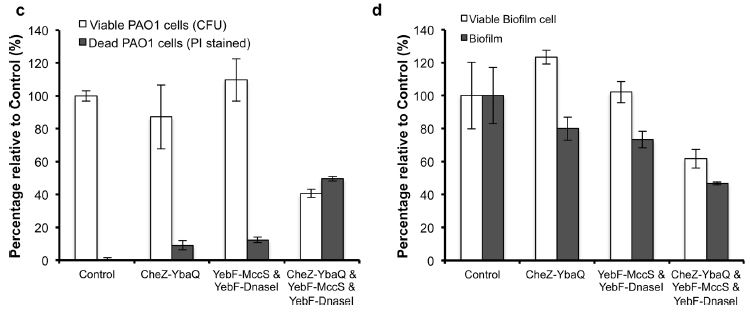
Finally, using a two-compartment tank separated by a porous membrane, they did an experiment where the P. aeruginosa and the E. coli started off on opposite sides. You can see quite clearly that adding the chemotactic ability by itself does not hurt the P. aeruginosa. Adding the killer cocktail by itself also does not significantly affect survival of the P. aeruginosa. But, the combination of chemotaxis, DnaseI to break down biofilm, and Microcin S to kill the cells combined to give an otherwise harmless E. coli the ability to sense, hunt down, and kill Pseudomonas.

No comments
Be the first one to leave a comment.