Fixing Dwarf Mice
Subject areas: Developmental Biology, Cell Biology
Vocabulary:
ossification – the final step in bone development, in which it hardens. Endochondral ossification happens in the long bones of the limbs, the vertebrae, sternum, and some parts of the skull. In endochondral ossification, cartilage forms first, and then it begins to form hardened bone starting from the middle of each bone and progressing outwards, replacing the cartilage as it goes. A second type of ossification, called intramembranous ossification, occurs without a cartilage intermediate. Progenitor cells start to cluster together, and as they do so, they differentiate into osteoblasts, they initiate the process of creating bone.
Wild-type – In experimental biology, especially when manipulating the genes of organisms, wild type (abbreviated WT) means a “normal” or reference organism. In the case here, wild-type mice are compared to mice with a mutation in the FGFR3 gene.
The article:
Garcia, S. et al. Postnatal soluble FGFR3 therapy rescues achondroplasia symptoms and restores bone growth in mice. Science Translational Medicine 5(203):1-10, 2013. doi:10.1126/scitranslmed.3006247
This article is very straightforward – they have a specific disease with a specific cause, and they hypothesize a specific treatment to overcome the primary symptoms of the disease.
The Setup
Achondroplasia is a rare genetic disease in humans, although it is the most common form of short limb dwarfism. Children with achondroplasia have impaired bone development, and thus are usually very short and may have bone deformities in the skull or vertebrae that cause neurological and orthopedic problems as well. There is no standard treatment for it, though limb-lengthening surgeries are sometime indicated, and treatment with human growth hormone has also been tested, without clear benefit.
We actually know exactly what causes achondroplasia. Patients are characterized with a single point mutation. That means within the approximately 16,500 nucleotides in the gene for FGFR3 (fibroblast growth factor receptor 3), just one specific change can lead to this achondroplasia. More specifically, it could be a change in either of two particular nucleotides, but either mutation does the same thing: it changes one specific amino acid in the receptor. (Remember, the DNA/gene is the information to make a sequence of amino acids, or a protein, which in this case is the FGFR3 receptor).
The FGFR3 receptor sits in the membrane of certain cells, like those involved in bone development, and when a growth factor molecule (FGF2, FGF9, or FGF18) comes along and binds to it, it activates for a while, turning on certain processes inside the cell, and then after a little while, lets go of the growth factor and turns off. The mutated FGFR3 receptor causing achondroplasia malfunctions stays on for too long after it is activated. At this time, it is unclear exactly how this affects bone development.
The Hypothesis
If the disease is caused by overactive FGFR3 receptors, it should be possible to counteract that by limiting the amount of FGF2, FGF9, and FGF18 available to activate the receptors in the first place. Totally knocking them out genetically would lead to other problems, since they are needed for normal function as well, so they needed to find a way to take away some of the active growth factors during a time important for bone growth, without disrupting their normal functions. They thought that if they could do that, then the problem of overactive FGFR3 receptors could be lessened or even eliminated.
The Experiment
Fortunately for these scientists, there happens to be a very good model system for achondroplasia. A mouse engineered with exactly the same mutation has all the essential symptoms of achondroplasia including small size, small limbs, some malformations of vertebrae leading to kyphosis (an abnormal humping curvature of the spine), and sometimes spinal cord compression/paralysis and airway obstruction. It’s a lot easier to get permission to experiment on mice than on human babies!
The mice were injected with soluble versions of a truncated FGFR3. Remember that normal FGFR3 is embedded in the cell membrane, and it binds a growth factor outside, then activates processes inside the cell. The injected soluble receptor, sFGFR3, will bind to growth factors that normally bind to FGFR3, but since it is not embedded in a cell, it does nothing. In effect, it is a decoy, temporarily binding up some of the available growth factors so that there is less activation of FGFR3.
What were they going to compare? Obviously, they want to compare normal mice to achondroplastic mice. They want to treat normal mice with the sFGFR3 at whatever concentrations they plan to use on achondroplastic mice to make sure there is no other effect. They want to use different concentrations because they have not tried this before, and the best experiment would use the minimal amount needed for an effect.
Of course, they would also want to compare injections of sFGFR3 with injections of solutions containing something similar but unable to bind growth factors to rule out non-specific effects from injecting a bunch of proteins into the animal.
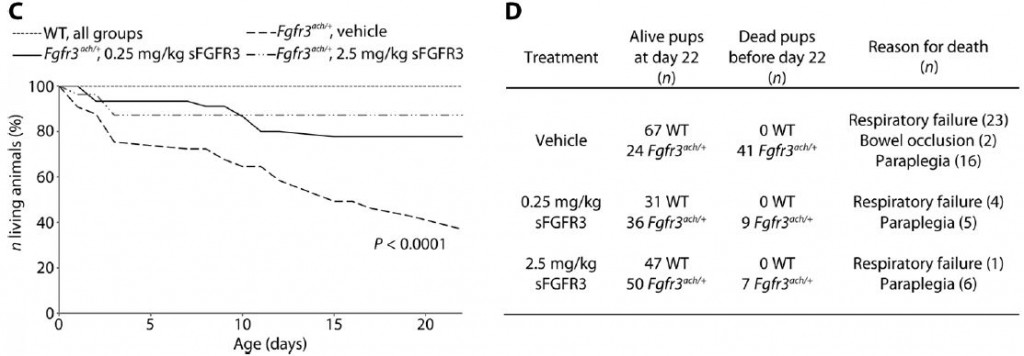
The graph and table below neatly summarize their findings on survival alone:
The treatment clearly has a significant effect in alleviating life-threatening symptoms in achondroplastic mice. It should be noted though that in humans, achondroplasia is rarely life-threatening, and most live a normal life-span. You can see that the sFGFR3 injection is harmless to the wild-type mice with none dead after 22 days. 63% of the mutant mice die within 22 days, but that drops to just 14% with the treatment!(WT=wild type mice, Fgfr3ach/+ are the achondroplastic mutants, “vehicle” refers to a sham injection missing the sFGFR)
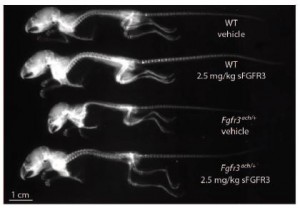
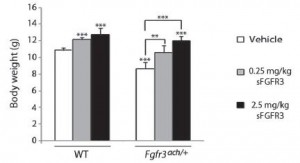
What about the other symptoms? The x-ray below shows that clearly the skeletal size is rescued to a normal size. The bar graph with it shows that overall body mass is improved as well.
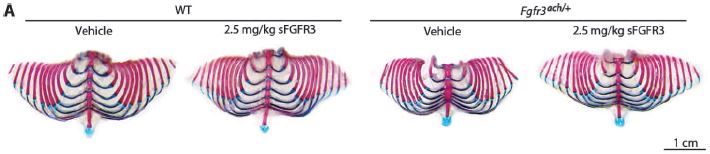
The next figure shows that rib cage development is also rescued, although not quite up to normal.
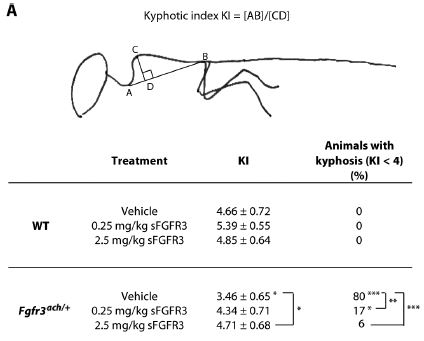 The figure to the right shows that the kyphosis (abnormal curvature of spine due to malformed vertebrae) can be significantly improved with the sFGFR treatment. They also showed that skull formation is improved, and also that there was no lasting detrimental effect of the treatment on fertility.
The figure to the right shows that the kyphosis (abnormal curvature of spine due to malformed vertebrae) can be significantly improved with the sFGFR treatment. They also showed that skull formation is improved, and also that there was no lasting detrimental effect of the treatment on fertility.
The Conclusion
All of this is strong support for the possibility of using sFGFR3 as a treatment for achondroplasia in humans. Before that happens though, they will need to work out more details with the mice. For these studies, they treated the mice for three weeks. It is not clear how the timing or length of treatment time affects the results of the treatment. Adjustments in optimal concentrations, more thorough examinations for unintended side effects, are all likely on the agenda for this research group.

No comments
Be the first one to leave a comment.