Sex and Healing of Degenerating Muscles
Subject areas: Cell Biology, Developmental Biology
Vocabulary:
Muscle-derived stem cells – A small subpopulation of muscle cells are able to proliferate and give rise to cells that can differentiate in different muscle cell types. In contrast, mature skeletal muscle cells do not proliferate.
muscular dystrophy – a group of diseases characterized by progressively worsening muscle weakness, caused by degeneration of the muscle cells. In the case of Duchenne Muscular Dystrophy, it is a genetic disease caused by a mutation in the dystrophin gene, but other dystrophies have different causes.
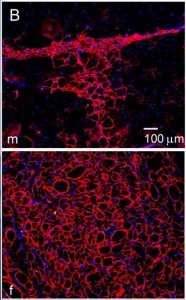
Extent of implanted male (top) and female (bottom) – muscle-derived stem cell contribution to regenerating dystrophic skeletal muscle.
The Article:
Deasy, B.M. et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J. Cell Biol. 177: 73-86 (2007). doi: 10.1083/jcb.200612094
This is an older article, published over six years ago, but I have come back to it for a couple of reasons. First, it was mentioned in an op-ed piece recently in Nature (Pollitzer, E. Nature 500:23-24, 2013) about the need to be cognizant of sex in research subjects. Second, I am interested in the science of sexual dimorphism, and this is a neat bit of work about something that would not be immediately thought of as having sex-specific differences.
Also, because we just covered an article about stem cells yesterday, there will be an assumption of passing familiarity with the concept.
What They Set Out To Do
In what is a fairly common story in scientific research, the most interesting finding was almost an accident. This research group had previously demonstrated that when muscle-derived stem cells (MDSCs) were injected into diseased muscles, a large number of muscle fibers would regenerate. They used a mouse model of Duchenne muscular dystrophy, and the results suggested a possible therapy. However, there was a lot of variation in the effectiveness of any given stem cell injection, so as good scientists, they wanted to figure out if there were any common threads linking those stem cells that were better at regeneration that might be different from those stem cells that were worse.
What They Found
As they compared the different cell types, they found a significant difference between MDSCs from female mice and MDSCs from male mice. The possibility of a difference between male and female cells was not unknown, and there is a large body of literature that shows many differences between males and females in non-reproductive-system cells or parts of the body. However, it is something that often gets lost, even now, when researchers are working at a cellular level. It is important to keep in mind that the genes on the X and Y chromosomes are not only expressed in sex-specific organs.
Once Deasy et al found the discrepancy between male and female muscle-derived stem cells, they proceeded to test the hypothesis that the chromosomal sex of the stem cell affects its ability to rescue dystrophic muscle. Looking at 25 populations of MDSCs, they found that stem cells derived from male muscle were significantly less effective at participating in skeletal muscle regeneration.
How Can They Explain This?
Does the sex of the dystrophic muscle matter, or just the sex of the stem cells? They selected two populations each of female- and male- MDSCs that all had very similar growth characteristics and expressed a similar level of several different genetic markers. What they found was that male-MDSCs had low regenerative abilities in both male and female host muscle tissues, but even within that low level, those in male hosts fared worse than those in female hosts. There was a similar result with female MDSCs. They regenerated better in both male or female hosts than the male MDSCs, but they did significantly better in female hosts than male hosts. So, matching the sex of stem cell and host muscle does not generally improve regeneration. Female skeletal muscle, both as host and as donor stem cells, regenerates better in all cases.
The next question was whether or not hormones have anything to do with it. Both male and female MDSCs were shown to have estrogen receptors (cells need receptors to respond to hormones). Oddly, while the male MDSCs were not affected, female MDSCs were actually worse at regeneration when stimulated with estrogen after implanting into muscle.
Surveying a broad array of genes in the stem cells to see if there were differences, they found that female MDSCs expressed more stress-related genes (genes that help deal with stress, not genes that cause stress). Checking 45 different stress-related genes, 29% were elevated in female cells, only 7% were elevated in male cells, and 64% were the same in muscle-derived stem cells of either sex.
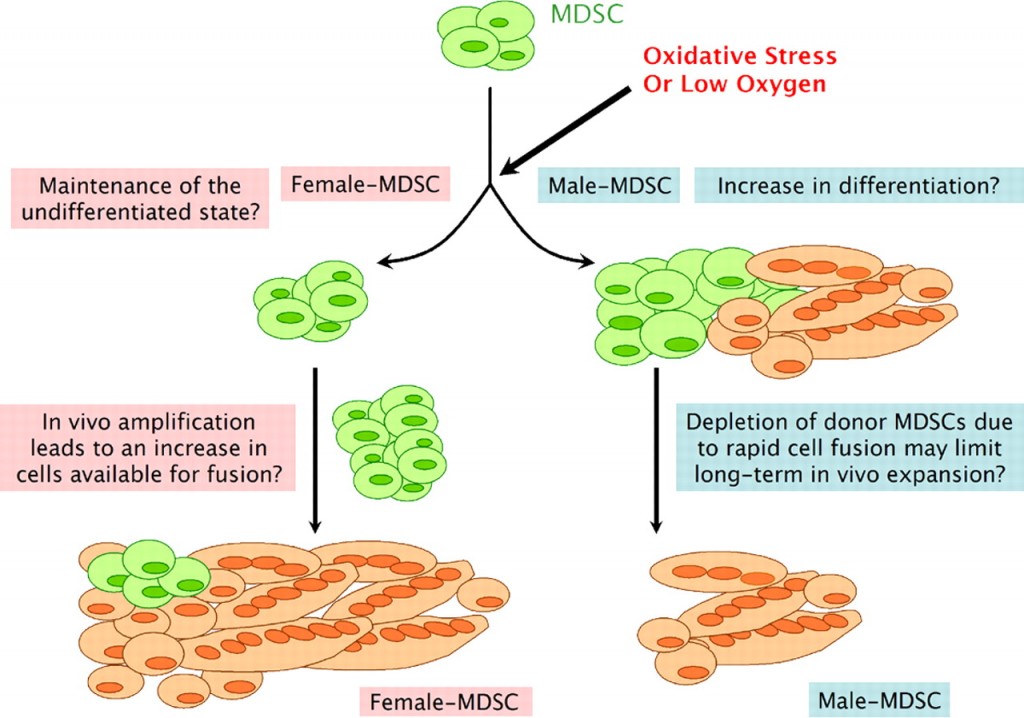
The scientists hypothesized that right after transplanting into the dystrophic muscle, the stem cells would be under stress, since other studies showed that inflammation occurred after stem cell transplant, and oxidative stress happens after inflammation and low oxygen. Interestingly, in conditions that promote differentiation from stem cells into muscle cells, they found that MDSCs from males actually differentiated significantly more under oxidative stress than the female MDSCs. That seems the reverse of what one might expect.
What is their Conclusion (so far)?
The research group’s explanation is interesting. Essentially, because the male MDSCs differentiate into muscle cells faster than the female cells in the stressful conditions of implantation into the diseased muscle, there are fewer stem cells available to help fix the muscle. The female MDSCs will proliferate first so there are more of them when they finally start to convert into muscle cells.
Postscript
A couple of years later, the same group found that unlike skeletal muscle, there is no sex-related difference between male or female muscle-derived stem cells when they are used to repair cardiac (heart) muscle.

No comments
Be the first one to leave a comment.